At human scale, controlling temperature is a straightforward concept. Turtles sun themselves to keep warm. To cool a pie fresh from the oven, place it on a room-temperature countertop.
At the nanoscale — at distances less than 1/100th the width of the thinnest human hair — controlling temperature is much more difficult. Nanoscale distances are so small that objects easily become thermally coupled: If one object heats up to a certain temperature, so does its neighbor.
When scientists use a beam of light as that heat source, there is an additional challenge: Thanks to heat diffusion, materials in the beam path heat up to approximately the same temperature, making it difficult to manipulate the thermal profiles of objects within the beam. Scientists have never been able to use light alone to actively shape and control thermal landscapes at the nanoscale.
At least, not until now.
In a paper published online July 30 by the journal ACS Nano, a team of researchers reports that they have designed and tested an experimental system that uses a near-infrared laser to actively heat two gold nanorod antennae — metal rods designed and built at the nanoscale — to different temperatures. The nanorods are so close together that they are both electromagnetically and thermally coupled. Yet the team, led by researchers at the University of Washington, Rice University and Temple University, measured temperature differences between the rods as high as 20 degrees Celsius. By simply changing the wavelength of the laser, they could also change which nanorod was cooler and which was warmer, even though the rods were made of the same material.
“If you put two similar objects next to each other on a table, ordinarily you would expect them to be at the same temperature. The same is true at the nanoscale,” said lead corresponding author David Masiello, a UW professor of chemistry and faculty member in both the Molecular & Engineering Sciences Institute and the Institute for Nano-Engineered Systems. “Here, we can expose two coupled objects of the same material composition to the same beam, and one of those objects will be warmer than the other.”
Masiello’s team performed the theoretical modeling to design this system. He partnered with co-corresponding authors Stephan Link, a professor of both chemistry and electrical and computer engineering at Rice University, and Katherine Willets, an associate professor of chemistry at Temple University, to build and test it.
Their system consisted of two nanorods made of gold — one 150 nanometers long and the other 250 nanometers long, or about 100 times thinner than the thinnest human hair. The researchers placed the nanorods close together, end to end on a glass slide surrounded by glycerol.
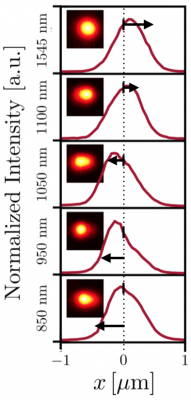
This figure shows evidence that the two nanorods were heated to different temperatures. The researchers collected data on how the heated nanorods and surrounding glycerol scattered photons from a beam of green light. The five graphs show the intensity of that scattered light at five different wavelengths, and insets show images of the scattered light. Arrows indicate that peak intensity shifts at different wavelengths, an indirect sign that the nanorods were heated to different temperatures. Bhattacharjee et al. ACS Nano 2019.
They chose gold for a specific reason. In response to sources of energy like a near-infrared laser, electrons within gold can “oscillate” easily. These electronic oscillations, or surface plasmon resonances, efficiently convert light to heat. Though both nanorods were made of gold, their differing size-dependent plasmonic polarizations meant that they had different patterns of electron oscillations. Masiello’s team calculated that, if the nanorod plasmons oscillated with either the same or opposite phases, they could reach different temperatures — countering the effects of thermal diffusion.
Link’s and Willets’ groups designed the experimental system and tested it by shining a near-infrared laser on the nanorods. They studied the beam’s effect at two wavelengths — one for oscillating the nanorod plasmons with the same phase, another for the opposite phase.
The team could not directly measure the temperature of each nanorod at the nanoscale. Instead, they collected data on how the heated nanorods and surrounding glycerol scattered photons from a separate beam of green light. Masiello’s team analyzed those data and discovered that the nanorods refracted photons from the green beam differently due to nanoscale differences in temperature between the nanorods.
“This indirect measurement indicated that the nanorods had been heated to different temperatures, even though they were exposed to the same near-infrared beam and were close enough to be thermally coupled,” said co-lead author Claire West, a UW doctoral candidate in the Department of Chemistry.
The team also found that, by changing the wavelength of near-infrared light, they could change which nanorod — short or long — heated up more. The laser could essentially act as a tunable “switch,” changing the wavelength to alter which nanorod was hotter. The temperature differences between the nanorods also varied based on their distance apart, but reached as high as 20 degrees Celsius above room temperature.
The team’s findings have a range of applications based on controlling temperature at the nanoscale. For example, scientists could design materials that photo-thermally control chemical reactions with nanoscale precision, or temperature-triggered microfluidic channels for filtering tiny biological molecules.
The researchers are working to design and test more complex systems, such as clusters and arrays of nanorods. These require more intricate modeling and calculations. But given the progress to date, Masiello is optimistic that this unique partnership between theoretical and experimental research groups will continue to make progress.
“It was a team effort, and the results were years in the making, but it worked,” said Masiello.
West’s co-lead authors on the paper are Ujjal Bhattacharjee, a former researcher at Rice University now at the Indian Institute of Engineering Science and Technology, Shibpur, and Seyyed Ali Hosseini Jebeli, a researcher at Rich University. Co-authors are Harrison Goldwyn and Elliot Beutler, both doctoral students in the UW Department of Chemistry; Xiang-Tian Kong and Zhongwei Hu, both research associates in the UW Department of Chemistry; and Wei-Shun Chang, a former research scientist at Rice, now an assistant professor of chemistry and biochemistry at the University of Massachusetts-Dartmouth. The research was funded by the National Science Foundation, the Robert A. Welch Foundation, and the University of Washington.
To learn more about Professor Masiello and his research program, please visit his faculty page and his research group website.
