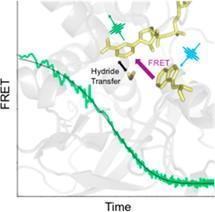
Enzyme catalysis is influenced by motions of the protein that alter the positions of reacting atoms in the active site. Convincing evidence has emerged from the laboratory of Judith Klinman (Univeristy of California, Berkeley) that spatially distinct regions of the protein control long-range motions that bring reactive atoms into the proper position for catalysis. Together with Klinman and Brian Dyer (Emory University), we have applied temperature-jump FRET (fluorescence energy transfer) spectroscopy to characterize the direction and lifetime of the activating motions. Thermophilic aldehyde dehydrogenase was employed, in which these motions are frozen out at a characteristic temperature. The activation enthalpy of the protein motions was found to determine the enthalpy of catalysis. Applications to other classes of enzymes are under design, in order to test the generality of these findings.
Enzyme dynamics from temperature-jump FRET lifetimes
Vaughn, M. B.; Zhang, J.; Spiro, T. G.; Dyer, R. B.; Klinman, J. P., Activity-related microsecond dynamics revealed by temperature-jump Förster resonance energy transfer measurements on thermophilic alcohol dehydrogenase. Journal of the American Chemical Society 2018, 140 (3), 900–903. https://pubs.acs.org/doi/10.1021/jacs.7b12369