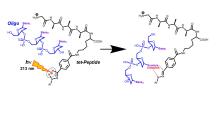
Gas-phase ion complexes of dinucleotides and trinucleotides with a diaryltetrazole-tagged peptide undergo covalent cross-linking upon UV photodissociation (UVPD) at 213 nm. The cross-linking reaction involves nitrile-imine intermediates produced by loss of N2 from the tetrazole, whereby cross-linking between the complex components competes with internal cross-linking within the peptide. The propensity for UVPD-induced cross-linking of DNA nucleobases was established for complexes of dinucleotides dAA, dCC, dGG, and dTT. UVPD-CID-MS3 of isomeric trinucleotide-peptide complexes of dCGA, dAGC, dCAG, dACG, and dGCA showed nearly quantitative cross-linking that favored guanine regardless of its position in the sequence. Binding energies for the gas-phase ion complexes were obtained by Born-Oppenheimer molecular dynamics and density functional theory calculations at the M06-2X/def2qzvpp level. These calculations showed similar binding energies for the dAA, dCC, and dCAG complexes that were in the 195-221 kJ mol-1 range, whereas binding to dGG and dTT was weaker. A mechanism for the novel cross-linking reaction between the nitrile imine and guanine was elucidated with a riboguanosine conjugate that was tagged with a diaryltetrazole group at 5’-O. Product analysis as well as the calculated structures and energies suggested that cross-links resulted from an attack on the aromatic ring of an imine intermediate by the guanine carbonyl oxygen, followed by proton migrations.
Covalent Crosslinking in Gas-Phase Biomolecular Ions. An Account and Perspective. Photochemical and Collision-Induced Crosslinking in Stereochemically Distinct Scaffolds of Peptides and Nitrile-Imine Conjugate Ions in the Gas-Phase
Turecek, F. Phys. Chem. Chem. Phys. 2023, 25, 32292-32304.
Zhu, H.; Nytka, M.; Vu, T. N. K.; Lemr, K. Turecek, F. J. Am. Soc. Mass Spectrom. 2024, 35, 3070-3088.
Vlk, M.; Wan, J.; Nytka, M.; Vu, T. N. K.; Lemr, K.; Tureček, F. J. Am. Soc. Mass Spectrom. 2025, 36, 175-186.
Wan, J.; Vlk, M.; Wei, C.; Turecek, F. J. Am. Soc. Mass Spectrom. 2025, 36, in press.